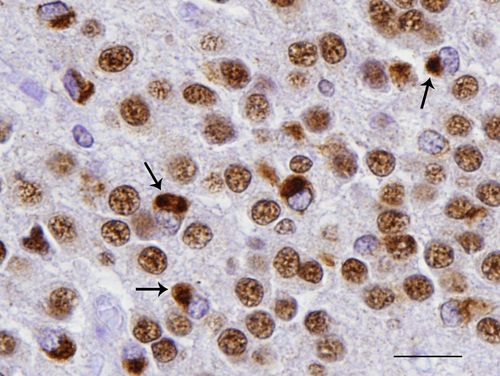
 Misfolded TDP-43 proteins in neurons of brain tissue (brown structures indicated by arrows) Credit: Felix Geser, MD, PhD, University of Pennsylvania School of Medicine.
Misfolded TDP-43 proteins in neurons of brain tissue (brown structures indicated by arrows) Credit: Felix Geser, MD, PhD, University of Pennsylvania School of Medicine.
Science, if anything, is incremental. It's a slow accumulation of knowledge punctuated by Eureka moments. As the years go by, one of my favorite aspects of working in science communications is watching how discoveries unfold - literally and figuratively, in the case of TDP-43, a normal protein that undergoes pathologic misfolding in the disease state.
As with all proteins, it is the shape of TDP-43 – the way the linear sequence of amino acids is ultimately folded into a three-dimensional protein – that is key to how it works, or doesn’t work. The normally folded TDP-43 protein is active mainly in the nucleus of cells throughout the body. It aids in editing the transcription of the genetic code.
A misfolded form of TDP-43 first became a major suspect in neurodegenerative diseases in late 2006, when the husband-and-wife team of Virginia M.Y. Lee, PhD and John Q. Trojanowski, MD, PhD, at Penn’s Center for Neurodegenerative Disease Research and Institute on Aging, made an important discovery: They found that mutated TDP-43 accumulated in post-mortem brain tissue from individuals diagnosed with certain types of frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS), or Lou Gehrig's disease. The misfolded, disease protein was recovered from only affected central nervous system regions, including the hippocampus, neocortex, and spinal cord. The approach that led to this finding was an unbiased study of proteins that behaved abnormally, in studies of FTLD cases first. Unexpectedly, they also found a form of TDP-43 in all of the ALS cases they subsequently studied.
To identify the protein, the group first made antibodies to the presumptive, misfolded disease protein, the identity of which they didn’t know at this stage of their studies. They then took brain extracts containing the mystery protein and injected it into mice, which then developed the monoclonal antibodies that recognize TDP-43. The researchers found that all 72 cases of FTLD or ALS examined contained misfolded TDP-43.
"Since many cases were studied, the data became very compelling," recalls Lee. Still, skepticism about TDP-43 being to blame for the pathology of ALS was part of the scientific discussion at the time.
Two years later, further proof that TDP-43 is the misfolded protein in ALS and FTLD emerged through findings that TDP-43 mutations track with disease. A flurry of reports, including one from Penn, showed that DNA isolated from brain tissue from ALS and FTLD patients harbored mutations in the gene that encodes TDP-43. Penn researchers surveyed 259 individuals with either ALS or ALS combined with FTD, with misfolded TDP-43 protein present, and determined the DNA sequence of the TDP-43 gene. They found two families in which a mutation was present. Within the same family, all members who have the disease carry the mutated form of TDP-43. The mutation was absent in unaffected individuals. Other groups made similar findings around the same time, lending greater strength to the evidence.
Says Trojanowski: “When all the mutations began to appear in 2008, investigators who expressed some doubts about our finding were won over.”
“A paradigm shift”
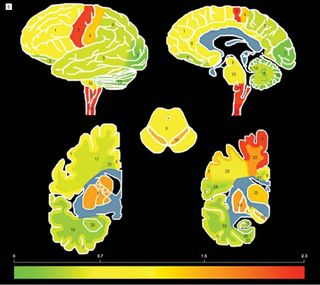 The emerging field of TDP-43 biology soon underwent a change in understanding how and where the diseases manifested themselves in the brain. Trojanowski and Lee showed that misfolded TDP-43 accumulates throughout the brain as well as the spinal cord of ALS patients, suggesting ALS has broader neurological effects than previously appreciated.
The emerging field of TDP-43 biology soon underwent a change in understanding how and where the diseases manifested themselves in the brain. Trojanowski and Lee showed that misfolded TDP-43 accumulates throughout the brain as well as the spinal cord of ALS patients, suggesting ALS has broader neurological effects than previously appreciated.
By again examining post-mortem brain tissue of ALS patients using TDP-43 specific antibodies, they observed effects not only in the areas of the brain and spinal cord that control voluntary movements, as expected based on the disease’s symptoms, but also in regions of the brain that involve cognition, executive functioning, memory, and involuntary muscle control.
So, as initially proposed in 2006, the new evidence supported the idea that ALS as well as ALS- PLUS [ALS with cognitive impairments] and FTLD all had the same underlying molecular pathology involving abnormal TDP-43.
"This constituted a paradigm shift in the way we think about these diseases," says Trojanowski.
At this point, researchers had firmly established TDP-43 as the culprit in some cases of ALS and FTLD, but what they didn’t know was how mutated TDP-43 might cause disease and what other genes and proteins played a role. This deepening of inquiry would take research to several different labs at Penn (and others) and at least three animal models.
More genetic factors affecting TDP-43
During his postdoc days at MIT, cell and developmental biologist Aaron Gitler, PhD, now an assistant professor at Penn, used a novel approach to screen for neurodegenerative disease genes using yeast cells, the same cells that bakers and brewers use to make bread and beer. Misfolded TDP-43 forms clumps in the simple yeast cells just like it does in human nerve cells. The clumping process takes decades to show up in humans but the researchers could model the process within a matter of hours in yeast cells. This allows for rapid genetic screening to identify proteins or even drugs that could potentially reverse harmful effects, and then testing the hits they find in animal models.
Using a combination of the yeast TDP-43 system and fruit flies, Gitler and Penn biologist Nancy Bonini, PhD, found evidence that mutations in the ataxin 2 gene were a genetic contributor to ALS cases associated with TDP-43 abnormalities. More specifically, the study showed that repeats of a bit of DNA encoding the amino acid glutamine in the ataxin 2 gene - a genetic stutter, as it were - are associated with an increased risk for ALS. The research began with Gitler's yeast screens in which genes were identified that could suppress or enhance TDP-43 toxicity. The team transferred 5,500 yeast genes into a strain of yeast they had been engineered to express misfolded human TDP-43. Among the genes that modified toxicity was the yeast counterpart of ataxin 2.
Gitler and Bonini transferred the genes to fruit flies to assess effects of the genes and their interactions in the nervous system. When the researchers directed expression of misfolded TDP-43 to the eye of the fruit fly, a progressive, age-dependent degeneration began. When directed to motor neurons, flies experienced a progressive loss of motility. Gitler, Bonini, and CNDR researchers then went on to show that people with the genetic stutter in their ataxin 2 gene had an increased risk for developing ALS.
But ataxin 2 wasn’t the only gene affecting misfolded TDP-43. Penn genetics researcher and pathologist Vivianna Van Deerlin, MD, PhD, led an international study using post-mortem brain tissue from 515 patients with TDP-associated FTLD, which found that these patients had multiple genetic variations called SNPs in common in a region on chromosome 7 containing the protein TMEM106B, compared to over 2,500 disease-free controls. From this, the team concluded that the TMEM106B gene variants confer a higher genetic risk for all FTLD-TDP patients, as well as in the subset of FTLD patients with disease-causing mutations in another protein called progranulin.
Beginning to understand how mutated TDP-43 causes disease
At the same time as some researchers were delving into the genetic evidence of TDP-43 and disease, biochemist James Shorter, PhD, was studying how TDP-43 misfolds at the protein level. Remarkably, in the absence of other molecular components, pure normal TDP-43 rapidly assembles into short soluble polymers called oligomers and aggregates that bear remarkable outward structural resemblance to the aggregates observed in the degenerating motor neurons of ALS patients. Shorter also mentions it’s important to note that normal – as well as mutated - TDP-43 forms aggregates in his system, and some TDP-43 mutants accelerate aggregation.
Shorter’s lab found that a section at one end of TDP-43’s amino acid sequence starts the misfolding and aggregation of pure TDP-43. This corroborated observations made by Aaron Gitler in yeast.
However, Shorter says a chance conversation between Shorter, Gitler and Oliver King at the Boston Biomedical Research Institute led to an unexpected twist in the investigation of TDP-43. Using a sophisticated bioinformatics approach, King recognized that the misfolding-initiating section of TDP-43 is remarkably similar to the type of section that enables some proteins to form prions in yeast. Prions are misfolded proteins implicated in mad cow disease in cattle and Creutzfeldt–Jakob disease in humans. Remarkably, virtually all of the mutations in TDP-43 that are linked to ALS lie in the prion-like section of TDP-43.
Shorter’s lab then established that in the context of the pure protein some ALS-TDP-43 mutations can accelerate aggregation, whereas other mutations do not. These findings meshed with other observations made by Gitler’s group in yeast, in which some ALS-linked TDP-43 mutations promote aggregation and toxicity, whereas others do not and result in proteins that are very similar to normal TDP-43. These data suggested that there is more than one way by which mutations promote ALS.
Shorter’s lab is now investigating methods to prevent or reverse the misfolding of TDP-43. Shorter notes, “The powerful combination of our pure protein biochemistry and Aaron Gitler’s approaches in yeast is likely to yield many new and profound insights into ALS, which will undoubtedly change the way we think about this disease.”
The latest chapter in the TDP-43 story adds yet another wrinkle. Just a few weeks ago, Lee showed the first direct evidence of how mutated TDP-43 can cause neurons to die in a mouse model. When human mutated-TDP-43 genes are put into the mice, mouse nerve-cells die because these cells stop producing enough normal mouse TDP-43. Since cells regulate the exact amount of TDP-43, over-expression of the human TDP-43 protein prevents the mouse TDP-43 from functioning normally.
Lee thinks this effect leads to neuron death in this model rather than clumps of TDP-43 because these clumps were rare in the mouse cells observed in this study. She says that it is not yet clear why clumps were rare in these mice when they are so prevalent in human post-mortem brain tissue of ALS and FTLD patients.
The researchers are now back to looking for more genetic partners for TDP-43 - specific genes that are regulated by TDP-43 and how messenger RNA (mRNA) is involved. TDP-43 stabilizes the structure of mRNA, in addition to other functions.
Knowing the genes involved in the normal function of TDP-43 will help researchers identify what goes awry when normal TDP-43 is missing or nonfunctional or clumps of misfolded TDP-43 crowd a cell’s interior.
The ongoing work on animal models is about to bear fruit as well: Fours years on from the publication of the original TDP-43 paper, Lee notes that CNDR will soon launch studies of strategies to prevent TDP-43-mediated nervous system degeneration using this mouse model of TDP-associated ALS and FTLD.
The saga of TDP-43 is really just beginning. The way data is coalescing around TDP-43 and multiple neurodegenerative diseases may indicate that the collective research effort is just starting to scratch the surface.
Related Links
Penn Medicine News Releases and Original Studies
News Stories