| > |
Researchers at the University
of Pennsylvania School of Medicine report how
the gene for utrophin, which codes for a protein very similar
to dystrophin, the defective protein in Duchenne muscular
dystrophy (DMD), puts the brakes on its own expression
in muscle cells, thereby suggesting a new target for treatment. |
| > |
Utrophin is normally made at the junction
where nerves meet muscles, an area called the neuromuscular
junction or synapse. In the present study, the Penn team
discovered that silencing is applied by a protein called
Ets-2 repressor factor (ERF) sitting on a small piece of
the utrophin gene called the N-box. |
| > |
The findings were published online in Molecular
Biology of the Cell, in advance of print publication. |
(PHILADELPHIA) – Researchers at the University
of Pennsylvania School of Medicine report how the
gene for utrophin, which codes for a protein very similar to
dystrophin, the defective protein in Duchenne
muscular dystrophy (DMD), puts the brakes on its own expression in muscle cells,
thereby suggesting a new target for treatment. The findings were
published online in Molecular
Biology of the Cell, in advance of print publication.
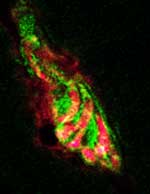 |
Expression of utrophin (green) at the synapse of normal skeletal muscles and nerves (red).
Click on thumbnail
to view full-size image |
The production of utrophin slows in fetal muscles soon after birth,
after which dystrophin takes over as the primary muscle-associated protein.
How this normal utrophin silencing occurs has been a mystery, until now.
If the brakes on utrophin production could be removed by drug intervention,
then increased utrophin expression could substitute for dystrophin as
a possible therapy for DMD, which affects 1 in 3,500 males.
Utrophin is normally made at the junction where nerves meet muscles,
an area called the neuromuscular
junction or synapse. In the present
study, the Penn team discovered that silencing is applied by a protein
called Ets-2 repressor factor (ERF) sitting on a small piece of the utrophin gene called the N-box.
“We demonstrated that ERF significantly reduces or represses the
activity of utrophin’s N-box in muscle cells of mice,” says
senior author Tejvir S. Khurana, MD, PhD, Associate Professor of Physiology and Member of the Pennsylvania
Muscle Institute. When the N-box was deleted
from the utrophin gene, ERF had no effect on silencing the utrophin gene,
as measured by an increase in utrophin gene-promoter activity. In another
experiment in which ERF was repressed, the researchers found
utrophin mRNA production increased.
“This approach of ‘repressing the repressor’ is medically
relevant to treating muscular dystrophy in that we hope to one day be
able to upregulate utrophin production,” explains Khurana.
Because utrophin is over 80 percent identical to dystrophin in its gene
sequence, utrophin could substitute for it in muscle cells. In normal
muscle cells dystrophin is part of a large complex of proteins that attaches
muscle cells to surrounding tissues. In DMD muscle cells, dystrophin
cannot perform this function and the muscles slowly fall apart. DMD patients
begin to have muscle
weakness and motor
difficulties as children, and
the condition worsens with age, eventually proving fatal around the third
decade of life.
“Dr. Khurana's work hints at what could be an important new drug
target for DMD – the more options we have with this disease, the
better,” says Sharon Hesterlee, PhD, Vice President for Translational
Research at the Muscular
Dystrophy Association. “We've known for
a while that increasing utrophin expression can reduce symptoms
of the disease, but it's very difficult to use a drug to increase
gene activity. What's nice about this work is that now we can try
to ‘block
a blocker’ to get the same effect – it's a more drug-friendly
approach.”
Other therapeutic strategies for DMD involve muscle-cell implantation,
stem-cell treatment, and gene
therapy. While there has been some progress
with these approaches, there have been many difficulties with graft vs.
host rejection and gene delivery. This new research suggests that blocking
ERF, either with drugs or by interfering with its RNA, may be more generally
feasible in most DMD patients.
There are several animal models of DMD, most notably the mdx mouse.
Khurana and his colleagues are currently investigating whether repressing
ERF in mdx mouse muscle reduces muscle deterioration.
“We have worked on this problem for a number of years, and our
current findings are a logical incremental step in understanding how
utrophin could become an effective tool for treating DMD,” states
Khurana. He cautions that while he hopes his work will lead to an effective
treatment someday, there are many steps and hurdles to get through first.
This work was funded in part by grants from the National
Institute of Arthritis and Musculoskeletal and Skin Diseases, the National
Eye Institute, the Muscular Dystrophy Association, and the Canadian
Institutes of Health Research. Co-authors on the study are Kelly
J. Perkins, Utpal Basu, Murat T. Budak, Caroline Ketterer, Santhosh
M. Baby, Olga Lozynska and Neal A. Rubinstein, from Penn, along
with John
A. Lunde and Bernard
J. Jasmin of the University
of Ottawa.
###
PENN Medicine is a $3.5 billion enterprise
dedicated to the related missions of medical education, biomedical
research, and excellence in patient care. PENN Medicine consists
of the University of Pennsylvania School of Medicine (founded
in 1765 as the nation's first medical school) and the University
of Pennsylvania Health System.
Penn's School of Medicine is ranked #2 in the nation for receipt
of NIH research funds; and ranked #3 in the nation in U.S. News
& World Report's most recent ranking of top research-oriented
medical schools. Supporting 1,400 fulltime faculty and 700 students,
the School of Medicine is recognized worldwide for its superior
education and training of the next generation of physician-scientists
and leaders of academic medicine.
The University of Pennsylvania Health System includes three hospitals,
all of which have received numerous national patient-care honors [Hospital
of the University of Pennsylvania; Pennsylvania Hospital, the nation's
first hospital; and Penn Presbyterian Medical Center]; a faculty practice
plan; a primary-care provider network; two multispecialty satellite
facilities; and home care and hospice.
Penn Medicine is one of the world’s leading academic medical centers, dedicated to the related missions of medical education, biomedical research, excellence in patient care, and community service. The organization consists of the University of Pennsylvania Health System and Penn’s Raymond and Ruth Perelman School of Medicine, founded in 1765 as the nation’s first medical school.
The Perelman School of Medicine is consistently among the nation's top recipients of funding from the National Institutes of Health, with $550 million awarded in the 2022 fiscal year. Home to a proud history of “firsts” in medicine, Penn Medicine teams have pioneered discoveries and innovations that have shaped modern medicine, including recent breakthroughs such as CAR T cell therapy for cancer and the mRNA technology used in COVID-19 vaccines.
The University of Pennsylvania Health System’s patient care facilities stretch from the Susquehanna River in Pennsylvania to the New Jersey shore. These include the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, Chester County Hospital, Lancaster General Health, Penn Medicine Princeton Health, and Pennsylvania Hospital—the nation’s first hospital, founded in 1751. Additional facilities and enterprises include Good Shepherd Penn Partners, Penn Medicine at Home, Lancaster Behavioral Health Hospital, and Princeton House Behavioral Health, among others.
Penn Medicine is an $11.1 billion enterprise powered by more than 49,000 talented faculty and staff.