The Tumor Response Assessment Core (TRAC) provides radiologic tumor response assessments using established tumor response criteria (ie. RECIST1.1) for patients enrolled in clinical trials at the University of Pennsylvania. These assessments are conducted by an experienced specialized group of radiologists at Penn Medicine who have a clinical and research focus in oncology.
Imaging for patients enrolled into IRB approved clinical trials conducted at Penn Medicine can be submitted to the TRAC for formal tumor response criteria assessments. These assessments do not take the place of a clinical report but complement the clinical report with formal response assessments.
TRAC tumor response reads not only provide quantitation of the tumor response but also provide reports that contain thumbnail images of what was measured and graphics of longitudinal target lesion burden over time.
Instructions for enrolling clinical trial patients into the TRAC
Step 1: Enroll the clinical trial with the TRAC by completing a Clinical Imaging Core (CIC) service center application. Please include the following information:
- Study title
- UPCC number
- IRB number
- Target accrual
- Tumor response criteria
- Ben financial number of funding source
Once your clinical trial is registered with the TRAC you will receive a confirmatory email indicating TRAC enrollment within 10 business days.
Step 2: Now that your clinical trial is enrolled, you can enroll your clinical trial patients. Before enrolling a patient, please ensure that patients meet the imaging criteria for evaluable disease.
For more information on lesion minimum size for commonly used tumor response criteria (select here button). To enroll your patient into the TRAC for an imaging read, use the EPIC codes you receive and order the TRAC read through EPIC:
- Baseline study
- Follow-up study
Information you will need when ordering a TRAC imaging read for a clinical trial patient:
- Dates and modalities of studies to be included in a given timepoint
- Date desired for completion of the TRAC reading
Understanding your TRAC Report
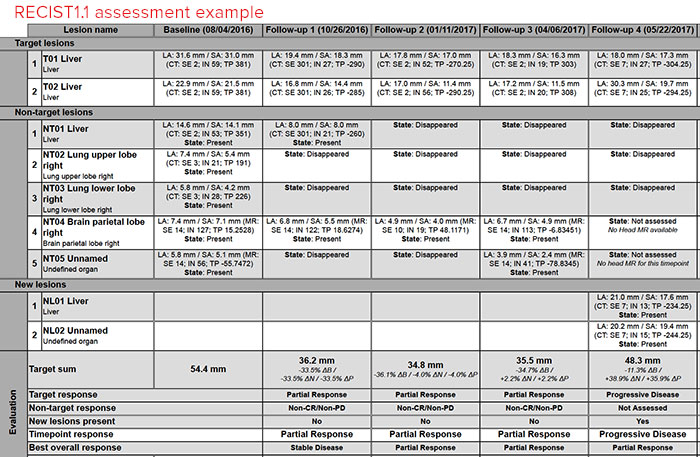
The TRAC report is generated using the response criteria that was selected during clinical trial enrollment. For each timepoint submitted, an assessment of percent change of target lesion burden and definition of that degree of change (complete response, partial response, stable disease, progression).
The TRAC report contains a detailed description of the measures of disease for each timepoint specific to the tumor response criteria employed in the assessment. Below is an example from a RECIST1.1 assessment for a patient on a clinical trial with liver metastasis:
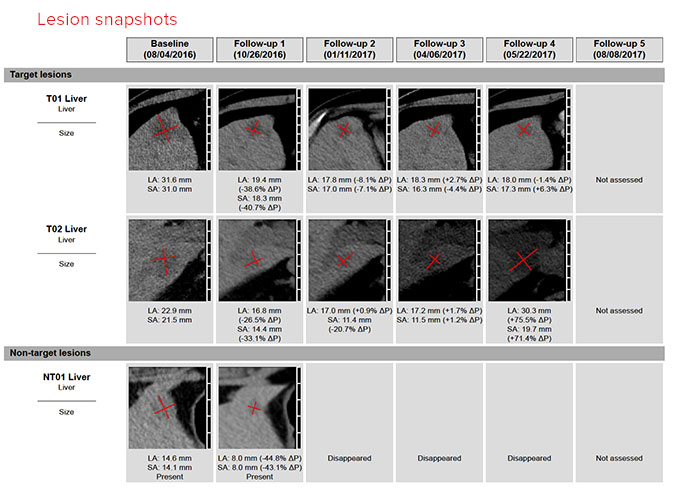
For each lesion included in the measurements, a thumbnail image of each of the measured lesions, along with the measurements used for the assessment, is provided in the report. This allows the clinical research team to view how lesions were measured and which lesions were selected for assessment.
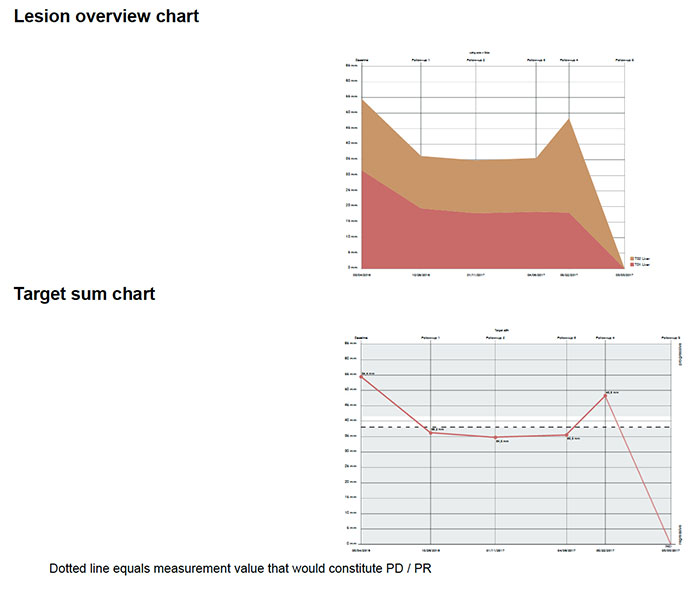
A response summary graphic is also provided to facilitate interpretation of changes in tumor lesions over time.
This TRAC tumor response report does not take the place of a clinical imaging read. TRAC reads are performed for research purposes only and are provided as a PDF in the patient’s EPIC chart. The patient’s clinical report will be addended to reference the accession number of the TRAC reading.
TRAC FAQ
Commonly asked questions and answers. Don’t see your question answered below? Please contact our core administrator Sarah Englander with any questions.
Does the TRAC read take the place of the clinical report?
No. The TRAC report is strictly for research purposes. Medical decisions are made from the clinical interpretation which is provided separately.
I need TRAC readings on the day the patient is seen in the office. Can I have this done in time for their office visits?
Yes. We had a core of radiologists working on TRAC reads daily. In order to be sure your patient TRAC reading is done by a specific time, please indicate when you need the TRAC reading and it will be prioritized accordingly.
Does the TRAC reading bill the patient?
No. The cost for TRAC reading is billed to the research funding source not to the patient.
Does the TRAC reading get billed to the Research Billing Account?
No. The cost for TRAC reading is billed through the Clinical Imaging Core service center.
Will the patient see their TRAC report on mypennmedicine?
No. The TRAC reading will be provided in the media section of the EPIC chart and will not be available to the patient through mypennmedicine.