At the OCRC we develop tumor vaccines using protein extracted from the whole fresh tumor obtained at the time of surgery. We have developed two distinct and complementary approaches. The first approach uses the patient's tumor to derive vaccines without prior knowledge of which antigens are expressed by the tumor. Although these antigens may not be known, they can still induce a potent immune response, because they can be effectively recognized by the immune system as "foreign" or "non-self". The second approach relies on the unique antigen profile of the patient's tumor. Each patient's tumor expresses unique proteins (antigens) that can be recognized and attacked by the immune system. This type of immunotherapy is highly personalized. Thus, immune therapy can be designed specifically for each patient, maximizing the potential for success.
The purpose of administering tumor vaccines is to boost pre-existing anti-tumor immune response as well as induce an immune response against new tumor antigens or in patients lacking spontaneous immunity.
There are currently two phase I/II clinical trials for patients with recurrent ovarian cancer using different approaches to manufacture personalized vaccine, developed from the patient's tumor:
 UPCC 19809: Uses protein (lysate) extracted from the patient's viably frozen tumor. The protein is loaded onto dendritic cells manufactured in the laboratory from the patient's blood cells. This personalized vaccine is administered every two weeks for a total of 5 times. The vaccine is now combined with bevacizumab, a drug commonly used to block angiogenesis (the growth of new blood vessels). Two weeks after the last injection, the patient undergoes a CT scan to assess the tumor burden.
UPCC 19809: Uses protein (lysate) extracted from the patient's viably frozen tumor. The protein is loaded onto dendritic cells manufactured in the laboratory from the patient's blood cells. This personalized vaccine is administered every two weeks for a total of 5 times. The vaccine is now combined with bevacizumab, a drug commonly used to block angiogenesis (the growth of new blood vessels). Two weeks after the last injection, the patient undergoes a CT scan to assess the tumor burden.
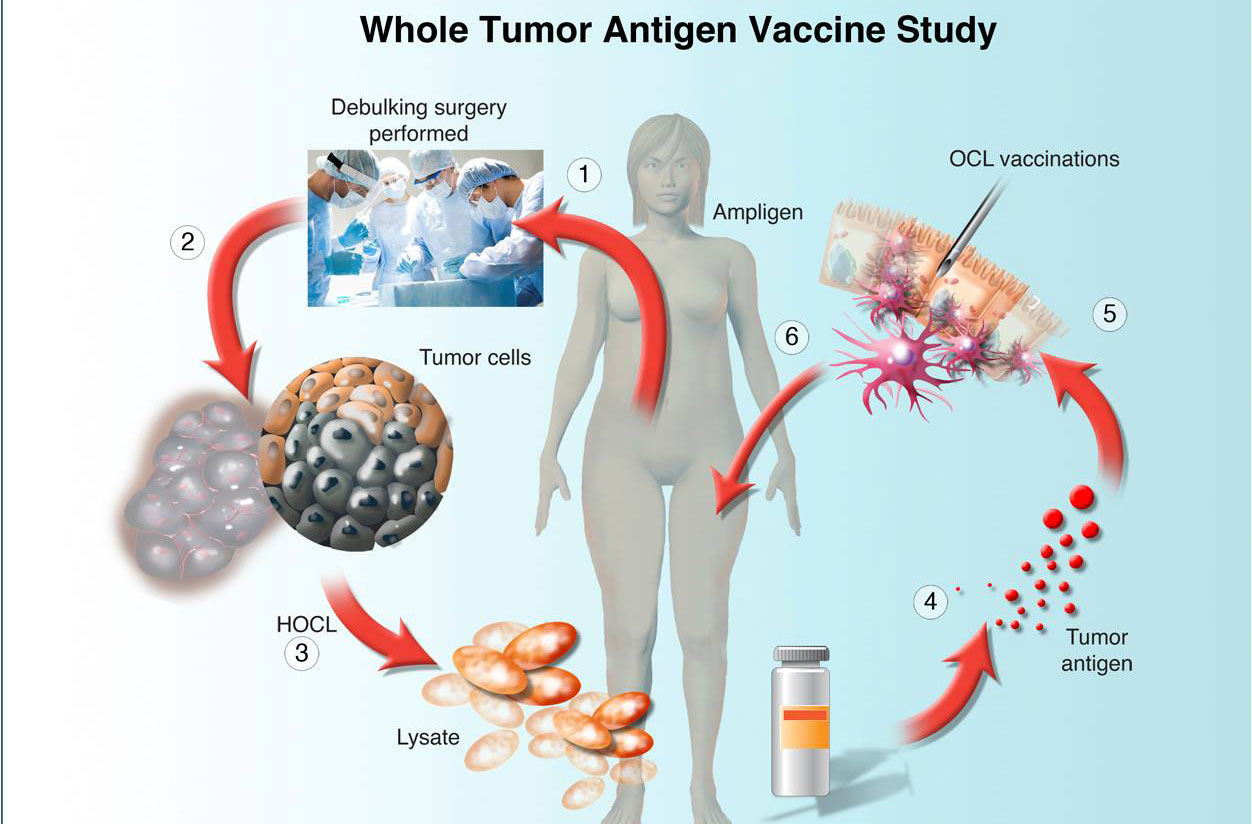 UPCC 29810: Uses protein (lysate) extracted from the patient's viably frozen tumor. The protein is given intradermally with tiny injections. Patients receive vaccine every two weeks for a total of 5 times. In some patients, vaccine is combined with intravenous Ampligen, to test if this agent will further activate the immune system and increase the effects of the vaccine. Ampligen is given every 2-3 days for three times after every vaccine. Two weeks after the last vaccine injection, the patient undergoes a CT scan to assess the tumor burden.
UPCC 29810: Uses protein (lysate) extracted from the patient's viably frozen tumor. The protein is given intradermally with tiny injections. Patients receive vaccine every two weeks for a total of 5 times. In some patients, vaccine is combined with intravenous Ampligen, to test if this agent will further activate the immune system and increase the effects of the vaccine. Ampligen is given every 2-3 days for three times after every vaccine. Two weeks after the last vaccine injection, the patient undergoes a CT scan to assess the tumor burden.
Although cancer vaccines (especially those using whole tumor antigen) are expected to have efficacy, amplifying the vaccine response can be accomplished through the various combinations described above or through adoptive lymphocyte therapy. Adoptive transfer of T cells has emerged as the most powerful approach to date for the treatment of patients with advanced malignancies. This is done by harvesting post-vaccine blood lymphocytes, activating them in culture, and infusing them back to the patient following a short course of high dose (non-myeloablative) chemotherapy.
 UPCC 26810: is a phase I trial currently enrolling patients who have completed vaccine studies. During the previous vaccine studies, we collect the patient's blood through a process called apheresis. Vaccine-activated T cells are isolated from the blood and stored viably until the patient is ready to enroll in this study. Once the patient enrolls, they receive three days of chemotherapy followed by infusion of her own activated lymphocytes. This approach is expected to boost the effects of the previous vaccines. Patients have the option of receiving additional maintenance vaccines.
UPCC 26810: is a phase I trial currently enrolling patients who have completed vaccine studies. During the previous vaccine studies, we collect the patient's blood through a process called apheresis. Vaccine-activated T cells are isolated from the blood and stored viably until the patient is ready to enroll in this study. Once the patient enrolls, they receive three days of chemotherapy followed by infusion of her own activated lymphocytes. This approach is expected to boost the effects of the previous vaccines. Patients have the option of receiving additional maintenance vaccines.
A large proportion of patients with ovarian cancer (approximately half) exhibit spontaneously anti-tumor immune response at the time of diagnosis. In these patients, tumor-reactive lymphocytes (T cells) recognizing the tumor can be isolated directly from their tumors at the time of surgery. The T cells are processed, stored and subsequently used for immunotherapy. When the patient is ready for immunotherapy, she receives three days of chemotherapy followed by infusion of her own activated lymphocytes in combination with cytokine therapy. The OCRC will launch this trial in early 2012.

In addition to the above methods, we are preparing to launch a series of trials involving adoptive T cell therapy using genetically modified T cells. Patient's blood lymphocytes are harvested through apheresis and engineered through the insertion of an antigen-specific artificial T cell receptor (called chimeric antigen receptor or CAR). The CAR-engineered T cells are thereby enabled to seek out a tumor antigen on the surface of ovarian cells and kill them. A large number of such CAR-engineered T cells can be expanded in culture and prepared for infusion. Once the patient enrolls, she receives three days of chemotherapy, followed by infusion of her own activated lymphocytes in combination with cytokine therapy. There are several known ovarian cancer associated antigens including the folate receptor- alpha, mesothelin and NY-ESO-1.
T-cells are engineered to target a specific receptor known as alpha folate receptor (aFR), which is over-expressed on 90 percent of ovarian cancers but not highly expressed by normal tissues. In pre-clinical tests, engineered cells were injected into the bloodstream of mice with established ovarian tumors. Dramatic anti-tumor responses were reproducibly achieved after the mice received an infusion of aFR genetically engineered T-cells. This exciting pre-clinical work was performed in Dr. Daniel Powell's laboratory at the OCRC. For more information on the preclinical work please visit http://www.uphs.upenn.edu/news/News_Releases/2011/08/ovarian-cancer.
We are preparing to launch this study at the end of 2011 and aim to recruit up to 21 patients with advanced recurrent ovarian cancer whose tumors express the alpha folate receptor.
The researchers of at the OCRC have also joined forces with the laboratory directed by Carl June, MD to launch two trials using genetically engineered T cells targeting mesothelin and NY-ESO-1.
