Synopsis: The perioperative management of patients with atrial fibrillation (AF) taking direct oral anticoagulants (DOAC) is a common clinical scenario, but for which best practices are unclear. The Perioperative Anticoagulation Use for Surgery Evaluation (PAUSE) study is a multi-center prospective cohort study of a standardized perioperative management approach of 3,007 adult patients with AF using apixaban, dabigatran or rivaroxaban undergoing elective surgery or procedure.
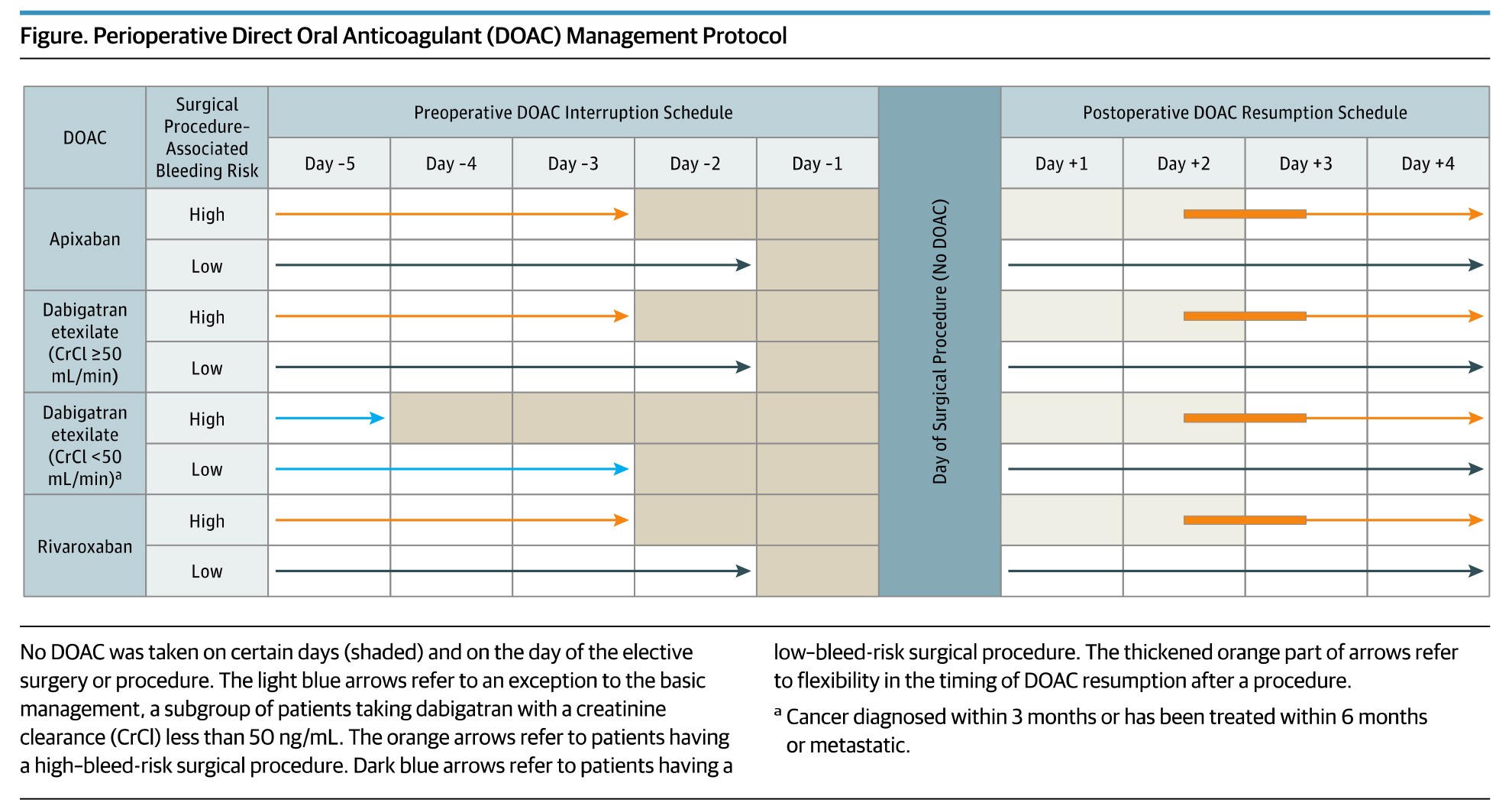
Preoperativley, the DOAC regimens were stopped 1 day before a low bleeding risk procedure and 2 days before a high bleeding risk procedure. Patients using dabigatran with impaired renal clearance had longer interruption intervals. Resumption of DOAC regimens occurred on post-operative day 1 (≈24 hours) for low bleeding risk procedures and day 2-3 (≈48-72 hours) for high bleeding risk procedures (see Figure).
Overall, the 30-day post-operative rate of major bleeding was 1.35% for apixaban (n=1,257 patients), 0.90% for dabigatran (n=668 patients) and 1.85% for rivaroxaban (n=1,082 patients). Among the 1,007 patients (33.5%) with a high bleeding-risk procedure, the rates of major bleeding were 2.96% for apixaban, 0.88% for dabigatran, and 2.95% for rivaroxaban. The rates of arterial thromboembolism were low across all three cohorts (apixaban 0.16%, dabigatran 0.60%, rivaroxaban 0.37%.
Clinical Takeaway:
In patients with AF on DOAC therapy, a simple, standardized approach to perioperative anticoagulation without heparin bridging or coagulation function testing is feasible and associated with low rates of major bleeding and arterial thromboembolism.
- Hornor MA, Duane TM, Ehlers AP, Jensen EH, Brown PS, Jr., Pohl D, et al. American College of Surgeons' Guidelines for the Perioperative Management of Antithrombotic Medication. Journal of the American College of Surgeons. 2018;227(5):521-36.e1.
This article provides additional guidance for perioperative management of antithrombotic medications, including warfarin and anti-platelet agents.