Synopsis: This is a case-series of 500 patients with total parenteral nutrition-dependent catastrophic and chronic gut failure who were referred for surgical intervention (particularly transplantation) at the Cleveland Clinic-Center for Gut Rehabilitation and Transplantation between 8/1/2012-2/15/2019.
Their objective was to define the evolving role of integrative surgical management including transplantation for patients with gut failure. They offered an algorithm for management of patients with gut failure which is guided by: 1. clinical status, 2. Splanchnic organ (hepatic) function, 3. anatomy of residual gut, and cause of gut failure (See image below). Noteworthy, they advocate for gut transplant first if a patient presents with gut failure and concomitant liver failure or non-reconstructable gut.
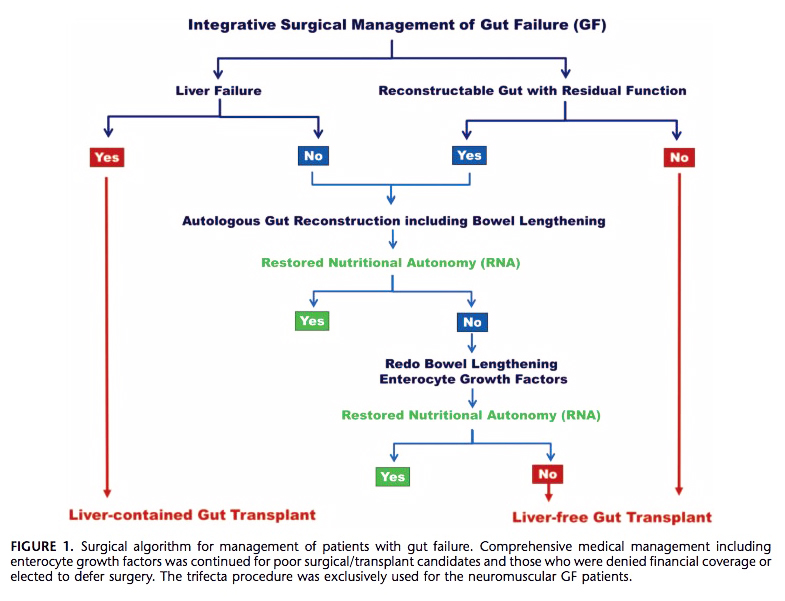
In their series, the mean age of patient was 45 ±17 years. Surgery was performed in 462 (92%) patients and 38 (8%) continued medical treatment. Definitive autologous gut reconstruction (AGR), which they defined as different reconstructive and remodeling procedures to reestablish gut continuity, restore normal alimentary flow, and modulate transit time, was achieved in 378 (82%) patients. Of the remainder, 42 (9%) had a primary transplant, and had 42 (9%) AGR followed by transplant. Among the transplant recipients, 27 (29%) had a liver along with the small bowel. There were a mean 1.9 autologous gut reconstructive surgeries per patient, and included primary reconstruction, interposition alimentary-conduits, intestinal/colonic lengthening, and reductive/decompressive surgery (check out the full text for lots of diagrams of surgeries and surgical options).
Overall patient survival was 86% at 1-year and 68% at 5-years with restored nutritional autonomy in 63% and 78%, respectively. Autologous gut reconstruction had better long-term survival than transplantation (74% vs 50% at 5-years, p =0.03). TPN therapy alone had the worst 5-year survival at 44%. Transplant was superior at restoring nutritional autonomy (83% in transplant recipients vs. 71% in reconstructive patients alone). The team proposed a model for predicting restored nutritional autonomy which includes anatomy of restored gut (<200 cm, no gut and presence of ileocecal valve), duration and calories of TPN, cause of gut-failure, and serum bilirubin (link to clinical model in paper).
Overall, surgical integration is an effective management strategy for gut-failure. The authors advocate for gut transplant only for patients who are not autologous gut reconstruction candidates (concurrent liver failure or non-reconstructable anatomy) or fail weaning of TPN therapy after multiple attempts at gut reconstruction. Of course, a main limitation of their data is confounding by indication but the Cleveland group’s experience show superior survival for patients who have reconstructive surgery alone.